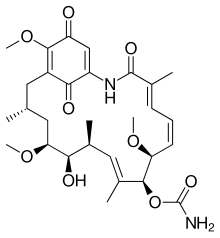
Ansamycins is a family of bacterial secondary metabolites that show antimicrobial activity against many Gram-positive and some Gram-negative bacteria, and includes various compounds, including streptovaricins and rifamycins.[1] In addition, these compounds demonstrate antiviral activity towards bacteriophages and poxviruses.
YouTube Encyclopedic
-
1/1Views:628
-
Rifamycin
Transcription
Structure
They are named ansamycins (from the Latin ansa, handle) because of their unique structure, which consists of an aromatic moiety bridged by an aliphatic chain.[2] The main difference between various derivatives of ansamycins is the aromatic moiety, which can be a naphthalene ring or a naphthoquinone ring as in rifamycin and the naphthomycins.[3] Another variation consists of benzene or a benzoquinone ring system as in geldanamycin or ansamitocin. Ansamycins were first discovered in 1959 by Sensi et al. from Amycolatopsis mediterranei, an actinomycete bacterium.[4]
Examples
Rifamycins are a subclass of ansamycins with high potency against mycobacteria. This resulted in their widespread use in the treatment of tuberculosis, leprosy, and AIDS-related mycobacterial infections.[5] Since then various analogues have been isolated from other prokaryotes.[citation needed]
References
- ^ Wehrli, W.; Staehelin, M. (1971). "Actions of the rifamycins". Bacteriological Reviews. 35 (3): 290–309. doi:10.1128/MMBR.35.3.290-309.1971. PMC 378391. PMID 5001420.
- ^ Prelog, V.; Oppolzer, W. (1973). "Rifamycins. 4. Ansamycins, a novel class of microbial metabolism products". 56: 2279.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Balerna, M.; Keller-Schierlein, W.; Martius, C.; Wolf, H.; Zähner, H. (1969). "Metabolic products of microorganisms. 72. Naphthomycin, an antimetabolite of vitamin K". Archiv für Mikrobiologie. 65 (4): 303–17. doi:10.1007/bf00412210. PMID 4988744. S2CID 31145406.
- ^ Sensi, P.; Margalith, P.; Timbal, M. T. (1959). "Rifomycin, a new antibiotic; preliminary report". Il Farmaco, Edizione Scientifica. 14 (2): 146–7. PMID 13639988.
- ^ Floss, H. G.; Yu, T. (1999). "Lessons from the rifamycin biosynthetic gene cluster". Current Opinion in Chemical Biology. 3 (5): 592–7. doi:10.1016/S1367-5931(99)00014-9. PMID 10508670.
