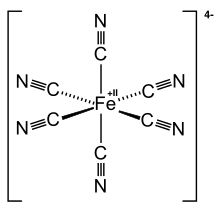
Anion in which a Fe2+ ion is complexed by 6 CN− ions
Identifiers
ChEBI
ChemSpider
KEGG
UNII
InChI=1S/6CN.Fe/c6*1-2;/q6*-1;+2
Key: UETZVSHORCDDTH-UHFFFAOYSA-N
[C-]#N.[C-]#N.[C-]#N.[C-]#N.[C-]#N.[C-]#N.[Fe+2]
Properties
C 6 Fe N 6 4−
Molar mass
−1
Related compounds
Related compounds
Ferricyanide
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Ferrocyanide is the name of the anion [Fe(CN )6 ]4− . Salts of this coordination complex give yellow solutions. It is usually available as the salt potassium ferrocyanide , which has the formula K4 Fe(CN)6 . [Fe(CN)6 ]4− is a diamagnetic species, featuring low-spin iron(II) center in an octahedral ligand environment . Although many salts of cyanide are highly toxic, ferro- and ferricyanides are less toxic because they tend not to release free cyanide.[1] Prussian blue and, as its potassium salt, an anticaking agent .[2]
YouTube Encyclopedic
1 / 5
Views: 2 484
7 517
2 389
305
2 375
There's Cyanide in Table Salt (But That's Okay!)
How to Write the Formula for Potassium ferrocyanide
Ferric Chloride and Potassium Ferrocyanide
Reactions Treatment of ferrocyanide with ferric-containing salts gives the intensely coloured pigment Prussian blue [1] ferric ferrocyanide and ferrous ferricyanide).
Ferrocyanide reversibly oxidized by one electron, giving ferricyanide :
[Fe(CN)6 ]4− ⇌ [Fe(CN)6 ]3− + e− This conversion can be followed spectroscopically at 420 nm , since ferrocyanide has negligible absorption at this wavelength while ferricyanide has an extinction coefficient of 1040 M−1 cm−1 .[3]
Applications The dominant use of ferrocyanides is as precursors to the Prussian blue pigments. Sodium ferrocyanide is a common anti-caking agent . Specialized applications involves their use as precipitating agents for production of citric acid and wine.[2]
Research Ferrocyanide and its oxidized product ferricyanide cannot freely pass through the plasma membrane . For this reason ferrocyanide has been used as a probe of extracellular electron acceptor in the study of redox reactions in cells . Ferricyanide is consumed in the process, thus any increase in ferrocyanide can be attributed to secretions of reductants or transplasma membrane electron transport activity.
Nickel ferrocyanide (Ni2 Fe(CN)6 ) is also used as catalyst in electro-oxidation (anodic oxidation ) of urea .[4] hydrogen production for cleaner energy with lower CO2 emission to wastewater treatment .
Ferrocyanide is also studied as an electrolyte in flow batteries .[5] [6]
Nomenclature According to the recommendations of IUPAC , ferrocyanide should be called "hexacyanidoferrate(II)". Cyanides as a chemical class were named because they were discovered in ferrocyanide. Ferrocyanide in turn was named in Latin to mean "blue substance with iron." The dye Prussian blue had been first made in the early 18th century. The word "cyanide" used in the name is from κύανος kyanos , Greek for "(dark) blue."
Gallery
Common ferrocyanide salts
Ag4Fe(CN)6
Ni4[Fe(CN)6]2
See also References
^ a b Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry . San Diego: Academic Press. ISBN 0-12-352651-5 ^ a b Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M.; Kellens, R.; Reddy, J.; Steier, N. (2011). "Cyano Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry doi :10.1002/14356007.a08_159.pub3 . ISBN 978-3527306732 ^ Appleby, C. A.; Morton, R. K. (1959). "Lactic dehydrogenase and cytochrome b2 of baker's yeast: Purification and crystallization" . Biochem. J . 71 (3): 492–499. doi :10.1042/bj0710492 . PMC 1196822 PMID 13638255 . ^ Geng, Shi-Kui; Zheng, Yao; Li, Shan-Qing; Zhao, Xu; Hu, Jun; Shu, Hai-Bo; Jaroniec, Mietek; Chen, Ping; Liu, Qinghua; Qiao, Shizhang (2020). "Nickel ferrocyanide as high-performance next generation electrocatalyst for urea oxidation". Nature Energy . doi :10.21203/rs.3.rs-67358/v1 . S2CID 231949301 . ^ Evans-Pritchard, Ambrose (2016-08-10). "Holy Grail of energy policy in sight as battery technology smashes the old order" . The Telegraph . ISSN 0307-1235 . Retrieved 2023-06-28 . ^ Developing Organic Flow Batteries for Energy Storage (arpa-e.gov) http://arpa-e.energy.gov/sites/default/files/documents/files/HarvardFlowBattery_OPEN2012_ExternalProjectImpactSheet_FINAL.pdf
Salts and covalent derivatives of the
cyanide ion
HCN
He
LiCN
<style data-mw-deduplicate="TemplateStyles:r1123817410">'"`UNIQ--templatestyles-0000002D-QINU`"'</style><span class="chemf nowrap">Be(CN)<sub class="template-chem2-sub">2</sub></span>
B(CN)<sub>3</sub>
C(CN)4 C2 (CN)2
NH4 CN ONCN O2 NCN N3 CN OCN− -NCO FCN
Ne
NaCN
Mg(CN)2 Al(CN)3 <link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Si(CN)<sub class="template-chem2-sub">4</sub></span>(CH3 )3 SiCN
P(CN)3 SCN− -NCS (SCN)2 S(CN)2 ClCN
Ar
KCN
Ca(CN)2
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sc(CN)<sub class="template-chem2-sub">3</sub></span>
Ti
V
Cr(CN)6 3−
Mn
Fe(CN)2 Fe(CN)6 4− Fe(CN)6 3−
Co(CN)2 Co(CN)3− 5
Ni(CN)2 Ni(CN)4 2− CuCN
Zn(CN)2 Ga(CN)3 <link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Ge(CN)<sub class="template-chem2-sub">2</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">As(CN)<sub class="template-chem2-sub">3</sub></span>(CH3 )2 AsCN (C6 H5 )2 AsCN
SeCN− BrCN
Kr
RbCN
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sr(CN)<sub class="template-chem2-sub">2</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Y(CN)<sub class="template-chem2-sub">3</sub></span>
Zr
Nb
Mo(CN)8 4−
Tc
Ru
Rh
Pd(CN)2 AgCN
Cd(CN)2 <link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">In(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sn(CN)<sub class="template-chem2-sub">2</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sb(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Te(CN)<sub class="template-chem2-sub">2</sub></span>
ICN
Xe
CsCN
Ba(CN)2 *
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Lu(CN)<sub class="template-chem2-sub">3</sub></span>
Hf
Ta
W(CN)<sub>8</sub><sup>4−</sup>
Re
Os
Ir
Pt(CN)4 2- AuCN Au(CN)2 -
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Hg<sub class="template-chem2-sub">2</sub>(CN)<sub class="template-chem2-sub">2</sub></span>Hg(CN)2
TlCN
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Pb(CN)<sub class="template-chem2-sub">2</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Bi(CN)<sub class="template-chem2-sub">3</sub></span>
Po
At
Rn
Fr
Ra
**
Lr
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Nh
Fl
Mc
Lv
Ts
Og
*
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">La(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Ce(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Pr(CN)<sub class="template-chem2-sub">3</sub></span>
Nd
Pm
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sm(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Eu(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Gd(CN)<sub class="template-chem2-sub">3</sub></span>
Tb
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Dy(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Ho(CN)<sub class="template-chem2-sub">3</sub></span>
Er
Tm
Yb(CN)3
**
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Ac(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Th(CN)<sub class="template-chem2-sub">4</sub></span>
Pa
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">UO<sub class="template-chem2-sub">2</sub>(CN)<sub class="template-chem2-sub">2</sub></span>
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
This page was last edited on 19 April 2024, at 16:18

![Fe4[Fe(CN)6]3](/wikipedia/commons/thumb/8/8d/Pigment_Berliner_Blau.JPG/120px-Pigment_Berliner_Blau.JPG)

![Ni4[Fe(CN)6]2](/wikipedia/commons/thumb/9/95/Nickel%28II%29_ferrocyanide.jpg/90px-Nickel%28II%29_ferrocyanide.jpg)
![K4[Fe(CN)6]](/wikipedia/commons/thumb/5/55/Potassium_ferrocyanide.jpg/90px-Potassium_ferrocyanide.jpg)