Harmala alkaloids are several alkaloids that increase effects of reward system neurotransmitter dopamine by acting as monoamine oxidase inhibitors (MAOIs). These alkaloids are found in the seeds of Peganum harmala (also known as Harmal or Syrian Rue), as well as leaves of tobacco[1] and coffee beans.[2] The alkaloids include harmine, harmaline, harmalol, and their derivatives, which have similar chemical structures, hence the name "harmala alkaloids". These alkaloids are of interest for their use in Amazonian shamanism, where they are derived from other plants. Harmine, once known as telepathine and banisterine, is a naturally occurring beta-carboline alkaloid that is structurally related to harmaline, and also found in the vine Banisteriopsis caapi. Tetrahydroharmine is also found in B. caapi and P. harmala. Dr. Alexander Shulgin has suggested that harmine may be a breakdown product of harmaline.[3] Harmine and harmaline are reversible inhibitors of monoamine oxidase A (RIMAs). They can stimulate the central nervous system by inhibiting the metabolism of monoamine compounds such as serotonin and norepinephrine.
The harmala alkaloids occur in Peganum harmala in concentrations of roughly 3%, though tests have documented anywhere from 2-7% or even higher,[4] as natural sources tend to vary widely in chemical makeup. Harmala alkaloids are also found in the Banisteriopsis caapi vine, the key plant ingredient in the sacramental beverage ayahuasca, in concentrations that range between 0.31 and 8.43% for harmine, 0.03-0.83% for harmaline and 0.05-2.94% for tetrahydroharmine.[5] Although other psychoactive plants are occasionally added to ayahuasca to achieve visionary states of consciousness, the recipes vary greatly and no single combination is common. Peganum harmala, normally consumed as a tea or used as an incense, is mentioned in classical Persian literature both as a sacred sacrament and as a medicine. The harmala alkaloids are not especially psychedelic, even at higher dosages, when hypnagogic visions, alongside vomiting and diarrhea, become the main effect.
Harmala alkaloids are also found in many other plants, such as Passiflora. The leaves of P. incarnata have been reported variously to give 0.005%, 0.12 mg%, and 0% harmala alkaloids.[6]
YouTube Encyclopedic
-
1/5Views:21 49410 5886 9138573 548
-
Trying To Extract Deadly Plant Alkaloids
-
Q&A 7 – Adderall Safety, Formaldehyde VS PCP, & Harmala Alkaloids (And More)
-
Harmaline
-
Harmala alkaloid | Wikipedia audio article
-
RGB Ripples - Syrian Rue (Peganum harmala) Trip Report #1
Transcription
Telepathine
Telepathine was originally thought to be the active chemical constituent of Banisteriopsis caapi, a key plant ingredient in the preparation of ayahuasca; a sacramental beverage from the Amazon.[7] This isolated chemical was so named because of the reported effects of ayahuasca among the indigenous users, including: collective contact with and/or visions of jaguars, snakes, and jeweled birds, and ancestral spirits; the ability to see future events; and as the name suggests, telepathic communication among tribal members.[8] It was assumed to be a newly discovered chemical at the time, however, it was soon realized that telepathine was already more widely known as "harmine" from its previous discovery in Peganum harmala (Syrian Rue).[9]
Uses

As mentioned above, some harmala alkaloids can be used as a monoamine oxidase inhibitor (MAOI) to facilitate the ingestion of dimethyltryptamine (DMT) and other tryptamines; while not generally used as a hallucinogen alone, there are reports of such use.[10] In high doses, it acts as purgative. Harmala alkaloids from Banisteriopsis caapi have been used to treat Parkinson's disease[citation needed]. As a benzodiazepine site inverse agonist, harmala alkaloids are used as a model for essential tremor (ET) when injected to animals. Rats being treated with harmaline exhibit severe tremors after 5–7 minutes. Individuals diagnosed with essential tremor have been found to have elevated blood levels of harmala alkaloids.[11]
Harmala alkaloids interact with smoked cannabis[12][13] when either smoked/vaporized, or taken orally as an extract or as a tea. Reports are scarce, but generally users experience more intense cannabis-like effects, as well as mild psychedelic effects such as hallucinations, ego dissolution, and increased emotions, especially in large doses.
Unlike MAOIs such as phenelzine, harmine and harmaline are reversible and selective meaning they do not have nearly as high a risk for "cheese syndrome" caused by consuming tyramine-containing foods, which is a risk associated with monoamine oxidase A inhibitors, but not monoamine oxidase B inhibitors.[14] Both MAO-A and MAO-B break down tyramine, but large doses of harmala alkaloids begin to affect MAO-B as well.
Anticancer
Isolated harmine was found to exhibit a cytotoxic effect on HL60 and K562 leukemic cell lines. This action might explain the previously observed cytotoxic effect of P. harmala on these cancer cells."[15]
Legal status
Australia
Harmala alkaloids are considered Schedule 9 prohibited substances under the Poisons Standard (October 2015).[16] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[16]
Exceptions are made when in herbs, or preparations, for therapeutic use such as : (a) containing 0.1 per cent or less of harmala alkaloids; or (b) in divided preparations containing 2 mg or less of harmala alkaloids per recommended daily dose.[16]
Chemical forms
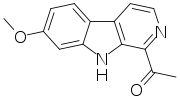
- Harmine: C13H12N2O
- 7-Methoxy-1-methyl-9H-pyrido[3,4-b]indole
- Harmine is a reversible inhibitor of monoamine oxidase A (RIMA).[17]
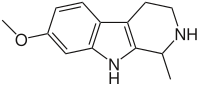
- Harmaline: C13H14N2O
- 4,9-Dihydro-7-methoxy-1-methyl-3H-pyrido[3,4-b]indole
- Harmaline is also a RIMA.[18]
- Harmalol: C12H12N2O
- 1-Methyl-4,9-dihydro-3H-pyrido[3,4-b]indol-7-ol
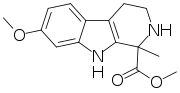
- Tetrahydroharmine: C13H16N2O
- 1,2,3,4-tetrahydro-harmine
- Harmalane: C12H10N2
- 1-Methyl-3,4-dihydro-beta-carboline. Harmalan occurs in foodstuffs.[19]
- Isoharmine: C13H12N2O[20]
- Harmine acid methyl ester:
- Harmilinic acid:
- Harmanamide:
- Acetylnorharmine:
See also
- Harmane
- Beta-carboline (norharmane)
- Monoamine oxidase inhibitor
- Reversible inhibitor of monoamine oxidase A (RIMA)
- Nicotiana rustica (Aztec tobacco)
- Hordenine
References
- ^ Kivell BM, Danielson K (2016). Neuropathology of Drug Addictions and Substance Misuse. Elsevier.
- ^ Herraiz T, Chaparro C (18 January 2006). "Human monoamine oxidase enzyme inhibition by coffee and beta-carbolines norharman and harman isolated from coffee". Life Sciences. 78 (8): 795–802. doi:10.1016/j.lfs.2005.05.074. PMID 16139309. Retrieved 21 November 2023.
- ^ Shulgin A, Shulgin A. "#13. HARMALINE". Tryptamines i Have Known And Loved: The Chemistry Continues. Erowid.
- ^ Herraiz T, González D, Ancín-Azpilicueta C, Arán VJ, Guillén H (March 2010). "beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO)". Food and Chemical Toxicology. 48 (3): 839–45. doi:10.1016/j.fct.2009.12.019. hdl:10261/77694. PMID 20036304.
- ^ Callaway JC, Brito GS & Neves ES (2005). Phytochemical analyses of Banisteriopsis caapi and Psychotria viridis Journal of Psychoactive Drugs 37(2): 145-150.
- ^ Dhawan K, Dhawan S, Sharma A (September 2004). "Passiflora: a review update". Journal of Ethnopharmacology. 94 (1): 1–23. doi:10.1016/j.jep.2004.02.023. PMID 15261959.
- ^ Djamshidian A, Bernschneider-Reif S, Poewe W, Lees AJ (January 2016). "Banisteriopsis caapi, a Forgotten Potential Therapy for Parkinson's Disease?". Movement Disorders Clinical Practice. 3 (1): 19–26. doi:10.1002/mdc3.12242. ISSN 2330-1619. PMC 6353393. PMID 30713897.
- ^ "Telepathine (ayahuasca) and psychic ability: field research in South America". British Psychological Society. Scarborough, UK. 20 September 2009.
- ^ "NCATS Inxight: Drugs". drugs.ncats.io. Retrieved 28 January 2020.
- ^ Shulgin, Alexander. "#13 Harmaline", Erowid Online Texts: TiHKAL #13 HARMALINE, retrieved November 26, 2006.
- ^ Louis ED, Zheng W, Jurewicz EC, Watner D, Chen J, Factor-Litvak P, Parides M (December 2002). "Elevation of blood beta-carboline alkaloids in essential tremor". Neurology. 59 (12): 1940–4. doi:10.1212/01.wnl.0000038385.60538.19. PMC 4992345. PMID 12499487.
- ^ "Syrian Rue & Cannabis - Erowid Exp - 'Intense, Terrifying and Unconcerning'". erowid.org. Retrieved 19 May 2022.
- ^ "Syrian Rue & Cannabis - Erowid Exp - 'Testing Its Power'". erowid.org. Retrieved 19 May 2022.
- ^ McKenna DJ, Callaway JC, Grob CS (1998). "The scientific investigation of Ayahuasca: a review of past and current research". The Heffter Review of Psychedelic Research. 1 (65–77): 195–223. Archived from the original on 8 October 2007. Retrieved 4 June 2007.
- ^ Jahaniani F, Ebrahimi SA, Rahbar-Roshandel N, Mahmoudian M (July 2005). "Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent". Phytochemistry. 66 (13): 1581–92. Bibcode:2005PChem..66.1581J. doi:10.1016/j.phytochem.2005.04.035. PMID 15949825.
- ^ a b c Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
- ^ Herraiz T, Guillén H (2018). "Monoamine Oxidase-A Inhibition and Associated Antioxidant Activity in Plant Extracts with Potential Antidepressant Actions". BioMed Research International. 2018: 4810394. doi:10.1155/2018/4810394. PMC 5820675. PMID 29568754.
- ^ Massaro EJ (2002). Edward J. Massaro, Handbook of Neurotoxicology. Humana Press. ISBN 978-0-89603-796-0. Retrieved 4 June 2020.[permanent dead link]
- ^ Herraiz T (November 2004). "Relative exposure to beta-carbolines norharman and harman from foods and tobacco smoke". Food Additives and Contaminants. 21 (11): 1041–50. doi:10.1080/02652030400019844. PMID 15764332. S2CID 216644379.
- ^ Ayoub Mikdad T., Rashan L.J. (1991). "Isoharmine, a β-carboline alkaloid from Peganum harmala seeds". Phytochemistry. 30 (3): 1046–1047. Bibcode:1991PChem..30.1046A. doi:10.1016/0031-9422(91)85312-N.
External links
- Tetrahydroharmine entry in TiHKAL • info
- "Peganum harmala (Zygophyllaceae) - Dr. Duke's Phytochemical and Ethnobotanical Databases at NAL". phytochem.nal.usda.gov. Archived from the original on 4 February 2023. Retrieved 11 January 2023.